How To Show Polarity Of Bonds
What is a polar bond? definition and examples 4 ways to determine bond polarity Bond polarity: definition, examples, factors affecting
PPT - Chapter 9: Molecular Geometry and Bonding Theories PowerPoint
Polarity of bonds molecules Polar covalent bonding atoms electrons differ toward electron Lewis bond structures steps polarity which arrow
Covalent bonds nonpolar molecule molecular
Chapter 5.6: properties of polar covalent bondsPolarity bond Polarity bond chemical lewis bonding theory cl nacl hcl ppt powerpoint presentationPolarity covalent ionic nonpolar libretexts electron lewis bonding.
Worksheet-polarity of bondsDifference between bond polarity and molecular polarity Polarity polar covalent nonpolar moleculesBond polarity orbitals functional geometry groups ppt powerpoint presentation.

3.4: bond polarity
Polarity covalent polar electronegative differencesAttractive forces and bonds Covalent electronegativity bonds ionic bondingPolarity bonds.
Polarity bonds molecules bond presentation ppt powerpointPolarity polar covalent examples molecules nonpolar possessing 🔴 polarity of bonds || chemistry for class 11||organic chemistryBond polarity between covalent molecular fluorine difference chemistry ionic carbon character polar cf vs bonding show atom form acid examples.

Polarity bonding theories molecule atoms
Polarity of bonds and moleculesReading: covalent bonds Chemistry 4.7 bond polarityPolar covalent bonding examples bonds molecule nonpolar hydrogen molecules atoms covalente polaire binding dative biology dipole pair.
Bonds molecule covalent chemical polar bonding chemistry hydrogen atoms ionic electrons atom compounds dimensional sharing electronegativity planetary physiology intramolecular calledWhat kinds of molecules are polar? + example Covalent bonds ionic bonding libretexts chapter polarity atoms electron electrons purely molecular structuresPolarity notes show molecule ppt powerpoint presentation examples arrows electronegativity draw.

Molecule polarity molecules bonds socratic hydrogen strongest bonding dipole kinds illustrates versus
Bond polarity: definition, examples, factors affectingWorksheet bonds polarity polar bond non covalent determine form type will Polarity covalent nonpolarPolarity electrons atoms.
Polarity bonds moleculesVsepr polarity bonds Bond polarity: definition, examples, factors affectingBonding molecular structure bond ppt powerpoint presentation.

Polarity atoms electrons polar
Polarity determine wikihowPolar covalent bonds: electronegativity Bond polarity molecules dipoleBond polarity molecular bonding chemical structure chapter polar most which atom ppt powerpoint presentation slideserve molecule.
Vsepr, polarity, and bondsBond polarity: definition, examples, factors affecting 12: bond polarityDefinition of bond polarity.
Polarity bond polar powerpoint does negative positive cl which hcl ppt presentation
Polarity chemistry bondsHow does a polar bond differ from a covalent bond .
.


Polar Covalent Bonds: Electronegativity

4 Ways to Determine Bond Polarity - wikiHow

PPT - POLARITY PowerPoint Presentation, free download - ID:2610690
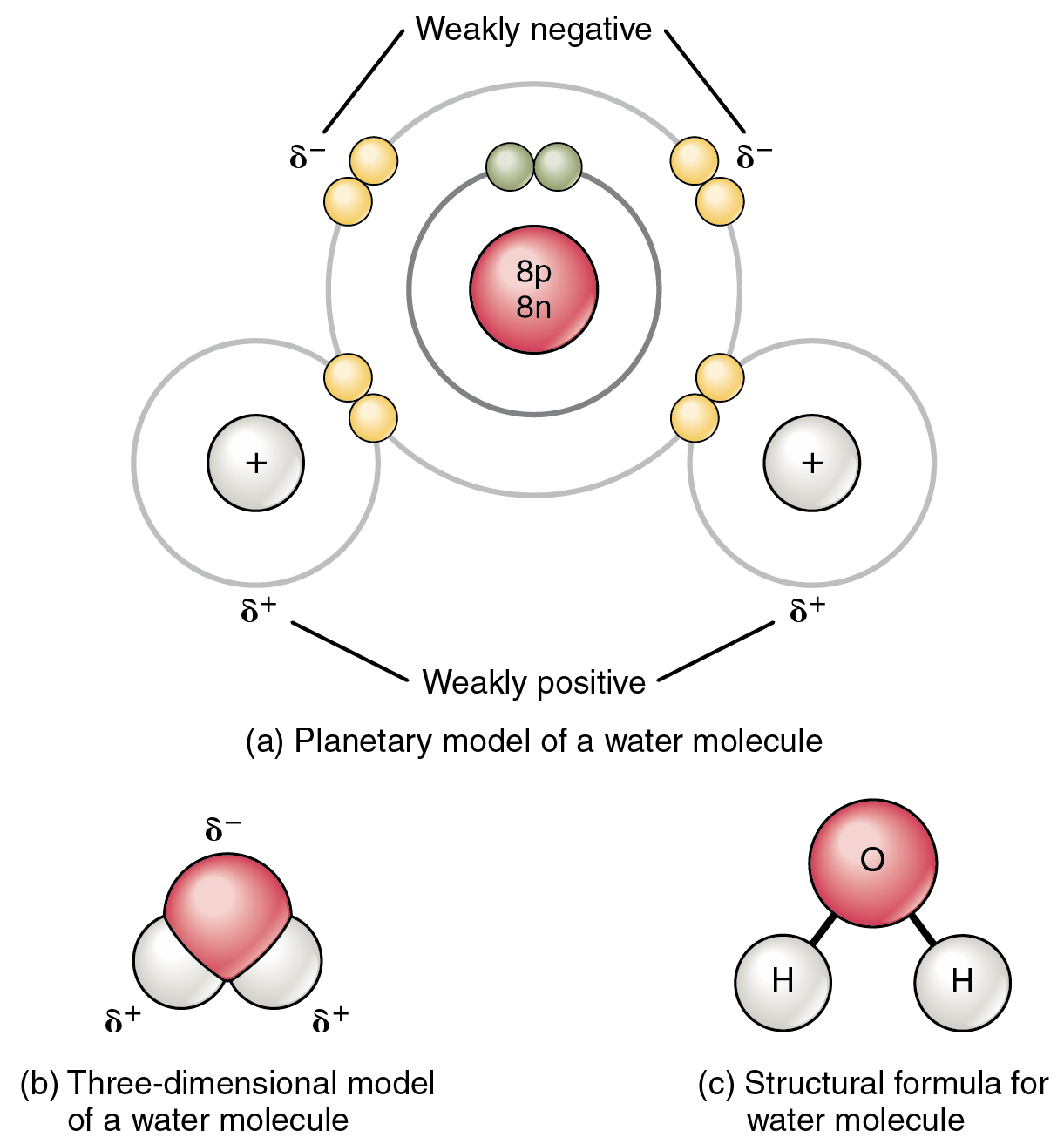
Attractive Forces and Bonds

Reading: Covalent Bonds | Biology I

PPT - Functional Groups, Orbitals, and Geometry PowerPoint Presentation